Table 1: Laser candidates for various medical applications.
Interaction of light (coherent or non-coherent) with cells may be categorized to:
(a) Endogeneous: direct absorbing by the constituents of the cells or tissue, and
(b) Exogeneous: light absorbed via the added photosensitizer, a process called photodynamics therapy (PDT) can be used to destroy cancerous or diseased cells and/or unwanted abnormal tissues.
PDT involves selective light (often low-power laser light or LED) absorption by the external chemical agent, or a PDT drug. As shown in Table 2, various dyes (drugs) have been developed at specific laser absorption wavelengths from visible to near-IR. The PDT drugs may be administered either intravenously or topically depending on applications. Three principal mechanisms have been proposed for the destruction of cells and tissues by PDT: localized cell damage by targeting on a specific organelle by a particular drugs, including apoptosis (localized in mitochondiria) and necrosis (localized in plasma membrane); vascular damage induced by PDT action. For example, the porphyrin-induced PDT produces a rapid onset of vascular blood flow stasis (stopping) and hemorrhage causing tumor cell death; and immunological response of PDT results a strong inflammatory reaction which contributes to tumor destruction.
Dermatology & Cancers
Table 2: Applications of photodynamic therapy (PDT).
The photochemical process of PDT for cell damage further involves 2 chemical processes: photoaddition reaction (type-I) in which the light-excited photosensitizer convalently bonds to a constituent molecule of the cell, where type I is the major path for crosslinking process; and photoxidation reaction (type-II) in which the excited state of the photosensitizer produces a highly reactive oxygen specie such as an excited singlet oxygen, a superoxide anion, or a free radical of H* and it often involves a chain reaction, where type II is the major path for cancer cell damage.
As shown in Table 2, PDT offers many applications in dermatology, ophthalmology and cancer treatments in various parts of human body.
As shown in Table 3, various photosensitizers are available for the absorption of lasers in visible (630-700) nm, and near-IR (700-1000) nm. These lasers are commercially available; dye lasers (at about 665 nm) pumped by a green laser; Nd:YAG (at 1064 nm); diode lasers (630-1100) nm; tunable Ti:sapphire laser (690-1100) nm; Alexandrite laser (720-800) nm. Tunable near-IR source may be also generated from an optical parametric oscillation (OPO) or amplification (OPA), where a green laser (at about 532 nm) may be used as a pump to produce tunable (900-1300) nm near-IR output (see Figure 3). In addition, high-brightness LED in visible (550-680) nm are also available for PDT using 5-ALA as the photosensitizer [24].

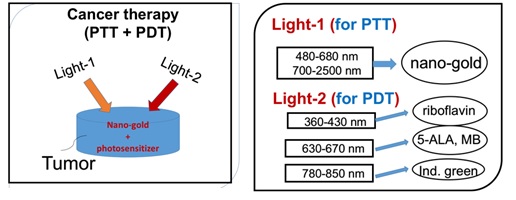
Figure 3. Combining PDT and PTT uisng nanogold and various photosensitizers [24].
Absorbing Extinction
Name of PDT drug
Photofrin (porfimer sodium)
Phthalocyanines (PTC), na-PTC
Indocyanin green (ICG)
Table 3: Summary of photosensitizers [24].
High intensity ultra-short pulse lasers have been used in PDT using a 2-photon absorption. These include: mode-locked Ti:sapphire laser (at 800 nm), peak power about 5MW/cm 2 , pulse duration 70 fsec and energy of 4 nJ/pulse; mode-lock Alexandrite laser (at about 780 nm) and a near IR-laser called Cr:forsterite (at 1230 nm). These near-IR lasers at longer wavelength offers deeper penetration than that of visible lasers, in addition to the minimal thermal effects and spatial selectivity which may be important in certain treatments such as brain cancers.
Laser penetration of tissues is limited by its absorption in melanin and oxyhomoglobin and it is a decreasing function of laser wavelength. Therefore near-IR lasers have deep penetration than visible lasers and have been used in various therapies which require deep penetrations, such as laser for hair removal and laser for acupunctures [6].
For ophthalmic application, retinal photocoagulation has been reported by using various visible and IR lasers such as argon blue-green laser (488/514 nm), double-YAG green laser (at 532 nm), krypton laser (at 647 nm) and diode lasers (at 806-810 nm). Photocoagulation process was also used to seal leak blood vessels for the treatment of age-related macular degeneration (AMD) which has two types, the choroidal neovascular (“CNV”, wet) and non-neovascular (dry). In the wet AMD, the vascular ingrowth causes photoreceptor destruction, or bleeding with extensive loss of vision. The major drawback of photocoagulation to destroy the vascular growth beneath the retina is the nonselective necrotic damage to the adjacent normal retina and the thermal damage in the subfocal area which can cause the recurrence of the neovascular tissue. However, a short-pulse (about 3 ns) green laser has been successfully used for the treatment of open-angle glaucoma, a procedure called selective laser trabeculoplasty (SLT) which shows advantages over the conventional system using an argon cw laser.
PDT for the treatment of subfocal choroidal neovascularization (CNV) using verteporfin as the phtosensitizer has been proven for subfoveal. CNV in both AMD and in pathologic myopia (or non-AMD) patients. CNV may be also treated by a procedure called transpupillary thermotherapy (TTT) using diode laser at 810 nm, where PDT drug is not needed.
In contrast to the PDT without too much heat involved, photothermal therapy (PTT) is a thermal process with heat generated by the thermal lasers. Examples of PTT for various applications in cancer therapy, cosmetic and dermatology are presented as follows.
Cancer therapy lasers
Combining the nanoparticles, diode lasers have been also used for cancer therapy [18], bio-sensing, bio-imaging, drug delivery and diagnostics of cancer cell [19-20]. Various nanoparticles (gold, polymers, silica etc) have been explored for the use of surface plasmon resonance (SPR) including shapes in spheres, rods, boxes, cages and shells [8-11]. For example, by changing the shape of nanogold from sphere to nanorod, the absorption and scattering peaks change from visible (about 530 nm) to the near-infrared (NIR) regime (about 750 to 980 nm) [18]. Comparing to the visible light, light in the NIR regime offers the advantages of larger absorption and scattering cross sections and much deeper penetration depth in tissues [18]. The in vivo studies in animal and/or human cancer therapy shall include the non-uniform gold nanorod (NGR) concentration in the tumor, the multi-layer normal-cancer tissue medium with multiple thermal parameters, and the blood flowing of the laser-targeted areas. The design of multiple-wavelengths laser system shall partially overcome the issues of GNRs non-uniform and multiple thermal medium for a 3-dimensional-therapy, in which various absorption penetration depths are available via the fiber-coupled multiple-wavelength laser simultaneously targeting the cancer tumors [18].
Combining PDT and PTT using nanogold and various photosensitizers for cancer therapy is shown in Figure 4 [24]. The critical factors of the synergistic therapy efficiency to be discussed include: the concentration of the initiator (nanogold or photosensitizers) in the treated medium, the wavelength, energy and the irradiation period of the light applied to the medium.
Cosmetic and dermatology lasers [1-9]
Figure 4 shows various cosmetic lasers and their applications for invasive and non-invasive uses defined by their tissue penetration depth (d). Lasers with small depth (d 2 mm) suitable for non-invasive simulation or hair removal. Figure 4 can be compared with Figure 2 for the penetration depth. Figure 4 shows the commercial laser for hair removal (using a diode laser at 810 nm with large penetration depth about 4 mm) and hair growth device (using a red, 635 -690 nm, LED or laser with smaller penetration depth and low power for surface stimulation). Figure 5 shows a UV (308 nm) light device for the treatment of psoriasis; and a pen-type blue laser (at 405 nm) combined with a red laser (at 660 nm) for the treatment of acne.
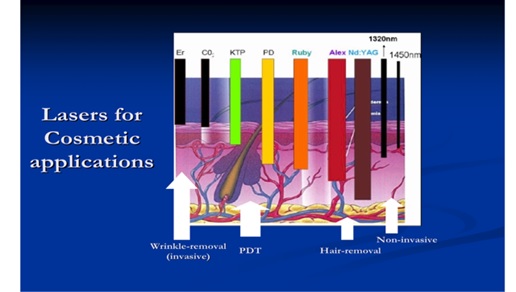
Figure 4. Cosmetic lasers and their tissue penetration depth which defined their applications in both invasive and non-invasive uses [5-9].
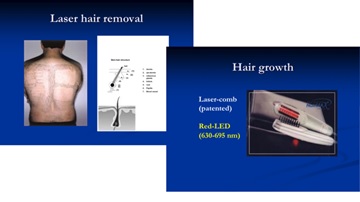
Figure 5. The commercial laser for hair removal (using a diode laser at 810 nm) and hair growth device (using a red, 635 - 690 nm LED or laser) [5,6].
Lasers (or LEDs) currently commercialized for dental applications include: (1) low power red laser (or LED, see Figure 6) activating methylene blue for antimicrobial photodynamic therapy (aPDT) to treat periodontal deceases; (2) diode laser (at 808 or 980 nm) for soft tissue cutting and stimulation; (3) Er:YAG laser, also called as “water-laser” (made by Biolase, US) for hard tissue cutting; (4) low power UV-blue laser (at 405 nm) for caries and cancer detection via light stimulated fluorescence.

Figure 6. (Left) a UV (308 nm) light for the treatment of psoriasis; (Right) a pen-type blue laser (at 405 nm) for the treatment of acne (made by New Vision Inc.) [25].
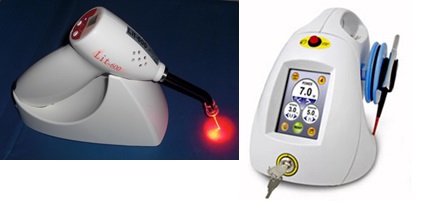
Figure 7. (Right) power red laser (or LED) for antimicrobial photodynamic therapy (aPDT) and (Left) diode laser (at 808 or 980 nm) for soft tissue cutting [25].
As shown by Table 3, various lasers have been used for various ophthalmic applications including retinal photocoagulation using argon blue-green laser (488/514 nm), double-YAG green laser (at 532 nm), krypton laser (at 647 nm) and diode lasers (at 806-810 nm). Photocoagulation process was also used to seal leak blood vessels for the treatment of age-related macular degeneration (AMD).
Figure 8 shows a surgical procedure called LASIK for vision corrections (myopia, hypeopia and astignism) using a UV excimer laser to reshape the corneal surface. Femto-second (f.s.) lasers combined with excimer laser are alo commercialized for the so-called bladeless LASIK procedure, in addition to the fetom-second laser cataracts treatment [16].
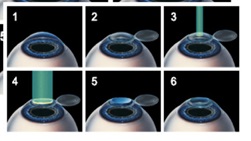
Figure 8. Surgical procedure called LASIK for vision corrections, where the corneal flap is prepare (either by microkeratom or a femtosecond laser), then an excimer UV laser (at 193 nm) is applied to cut a thin surface layer of the central part of the stroma tissue to reshape its curvature (for myopic correction); the corneal flat is placed back for healing [15,16].
Figure 9 shows a patented system using a solid-state (213 nm) laser for PRK and LASIK in replacing the gas excimer laser, where nonlinear crystals are sued to convert the 1064 nm laser to its fifth harmonics at 213 nm. Figure 10 shows a scanning Lasik system using a small flying spot for customized vision corrections.
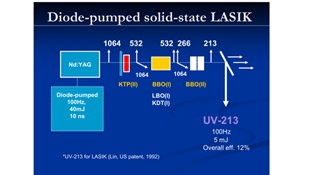
Figure 9. shows a patented system using a solid-state (213 nm) laser for PRK and LASIK (JT Lin, US 1992 patent) [14].
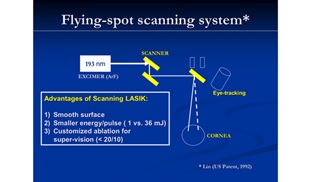
Figure 10. shows a scanning Lasik system using a small flying spot for customized vision corrections (JT Lin, US patent, 1993) [14-16].
Figure 11 shows a diode laser (at 808 or 980 nm) for photocoagulation of the corneal tissue for glaucoma treatment. Figure 12 shows two systems (made by New vision Inc. and MLase AG) using UV LED (at 365 nm) for corneal collagen crosslinking (CXL) for the treatment of keratoconus and other corneal deceases [17].

Figure 11. shows a diode laser (at 808 or 980 nm) for photocoagulation of the corneal tissue for glaucoma treatment, where a probe tip connected to the fiber is used to deliver the laser energy [25].

Figure 12. shows two systems (made by MLase AG, Germany, top; and New vision Inc. , Taiwan, low) using UV LED (at 365 nm) for corneal collagen crosslinking (CXL). Also shown is a pen-type CXL specially designed for VET uses [25].
Applications of medical lasers are characterized by the properties of the tissues and the matching absorption wavelengths. For PDT procedures, the properties of the activated photosensitizers also play an important role. Synergistic therapy efficiency may be improved by combining PDT and PTT using nanogold and various photosensitizers. Recent new technology also combines the nanomateirials for improved clinical outcomes.