Artificial intelligence (AI), particularly generative AI, has emerged as a transformative tool in healthcare, with the potential to revolutionize clinical decision-making and improve health outcomes. Generative AI, capable of generating new data such as text and images, holds promise in enhancing patient care, revolutionizing disease diagnosis and expanding treatment options. However, the utility and impact of generative AI in healthcare remain poorly understood, with concerns around ethical and medico-legal implications, integration into healthcare service delivery and workforce utilisation. Also, there is not a clear pathway to implement and integrate generative AI in healthcare delivery.
This article aims to provide a comprehensive overview of the use of generative AI in healthcare, focusing on the utility of the technology in healthcare and its translational application highlighting the need for careful planning, execution and management of expectations in adopting generative AI in clinical medicine. Key considerations include factors such as data privacy, security and the irreplaceable role of clinicians’ expertise. Frameworks like the technology acceptance model (TAM) and the Non-Adoption, Abandonment, Scale-up, Spread and Sustainability (NASSS) model are considered to promote responsible integration. These frameworks allow anticipating and proactively addressing barriers to adoption, facilitating stakeholder participation and responsibly transitioning care systems to harness generative AI’s potential.
Generative AI has the potential to transform healthcare through automated systems, enhanced clinical decision-making and democratization of expertise with diagnostic support tools providing timely, personalized suggestions. Generative AI applications across billing, diagnosis, treatment and research can also make healthcare delivery more efficient, equitable and effective. However, integration of generative AI necessitates meticulous change management and risk mitigation strategies. Technological capabilities alone cannot shift complex care ecosystems overnight; rather, structured adoption programs grounded in implementation science are imperative.
It is strongly argued in this article that generative AI can usher in tremendous healthcare progress, if introduced responsibly. Strategic adoption based on implementation science, incremental deployment and balanced messaging around opportunities versus limitations helps promote safe, ethical generative AI integration. Extensive real-world piloting and iteration aligned to clinical priorities should drive development. With conscientious governance centred on human wellbeing over technological novelty, generative AI can enhance accessibility, affordability and quality of care. As these models continue advancing rapidly, ongoing reassessment and transparent communication around their strengths and weaknesses remain vital to restoring trust, realizing positive potential and, most importantly, improving patient outcomes.
Artificial intelligence (AI) has become an increasingly popular tool in a variety of fields, including healthcare, with the potential to transform clinical decision-making and improve health outcomes [1,2,3]. Generative AI is one area of AI that has gained attention recently for its ability to use machine learning algorithms to generate new data, such as text, images and music [4,5,6,7]. Generative AI is proving to be a change catalyst across various industries, and the healthcare sector is no exception [8]. With its remarkable ability to analyse extensive datasets and generate valuable insights, generative AI has emerged as a powerful tool in enhancing patient care [9], revolutionizing disease diagnosis [10] and expanding treatment options [11]. By harnessing the potential of this cutting-edge technology, healthcare professionals can now access unprecedented levels of accuracy, efficiency and innovation in their practices.
Despite the potential benefits, the utility and impact of generative AI in healthcare remain poorly understood [12, 13]. The application of generative AI in healthcare raises ethical and medico-legal concerns [14]. Moreover, it is unclear how generative AI applications can be integrated into healthcare service delivery and how the healthcare workforce can utilise them appropriately [15]. Furthermore, it is uncertain how far generative AI can improve patient outcomes and how this can be assessed. Finally, the value of generative AI beyond augmenting clinical and administrative tasks needs to be explored.
Realizing generative AI’s vast potential in healthcare requires translational approaches rooted in implementation science. Such approaches recognize technological progress alone will not revolutionize healthcare overnight [16, 17]. Real change requires carefully orchestrated sociotechnical transitions that put people first. Implementation science-based approaches provide generalizable roadmaps grounded in empirical evidence from prior health IT deployments [16]. As such, healthcare leaders pioneering generative AI integration would be well served in leveraging these models to reinforce patient safety, trust and impact [17]. To facilitate the appropriate incorporation and application of generative AI in healthcare, this article aims to provide an overview of the use of generative AI in healthcare followed by guidance on its translational application.
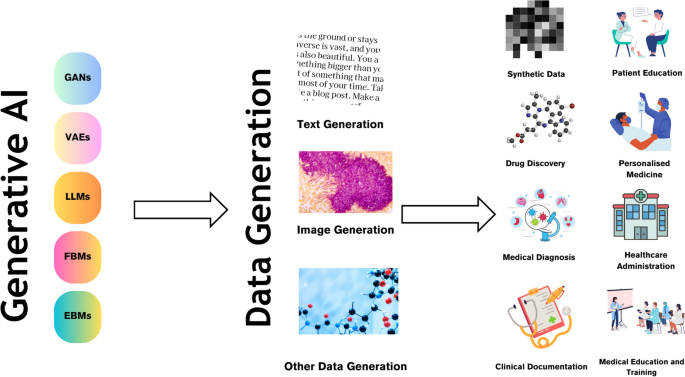
Generative AI is a class of machine learning technology that learns to generate new data from training data [18, 19]. Generative models generate data that is similar to the original data. This can be useful in a variety of applications such as image and speech synthesis. Another unique capability is that they can be used to perform unsupervised learning, which means that they can learn from data without explicit labels [8]. This can be useful in situations where labelled data is scarce or expensive to obtain. Furthermore, generative AI models can generate synthetic data by learning the underlying data distributions from real data and then generating new data that is statistically similar to the real data. Generative models differ from other types of machine learning models in that they aim to endow machines with the ability to synthesise new entities [8]. They are designed to learn the underlying structure of a dataset and generate new samples that are like the original data. This contrasts with discriminative models, which are designed to learn the boundary between different classes of data. These models focus on tasks such as classification, regression or reinforcement learning, where the goal is to make predictions or take actions based on existing data. There are several categories of generative AI, as outlined in Table 1 [20,21,22,23].

Synthetic data, which is created using generative AI models like GANs, is an increasingly promising solution for balancing valuable data access and patient privacy protection [9]. By using generative AI models, realistic and anonymised patient data can be created for research and training purposes, while also enabling a wide range of versatile applications. Moreover, GANs can synthesise electronic health record (EHR) data by learning the underlying data distributions, which allows for excellent performance and addresses challenges such as data privacy concerns. This approach can be particularly useful in situations where there is a limited amount of real-world patient data available, or when access to such data is restricted due to privacy concerns. Additionally, the use of synthetic data can help to improve the accuracy and robustness of machine learning models, as it allows for a more diverse and representative range of data to be used in the training process. Furthermore, the ability to generate synthetic data with different characteristics and parameters can enable researchers and clinicians to investigate and test various hypotheses [5, 9, 37], leading to new insights and discoveries.
Generative AI models are also being used to generate novel small molecules, nucleic acid sequences and proteins with a desired structure or function, thus aiding in drug discovery [11]. By analysing the chemical structure of successful drugs and simulating variations, generative AI can produce potential drug candidates at a much faster rate than traditional drug discovery methods. This not only saves time and resources but can also help to identify drugs that may have gone unnoticed using traditional methods. Moreover, the use of generative AI can also aid in predicting the efficacy and safety of new drugs, which is a crucial step in the drug development process. By analysing vast amounts of data, generative AI can help to identify potential issues that may arise during clinical trials, which can ultimately reduce the time and cost of drug development [11, 38]. In addition, generative AI by identifying specific biological processes that play a role in disease can help to pinpoint new targets for drug development, which can ultimately lead to the development of more effective treatments.
Generative models can be trained on vast datasets of medical records and imagery (like MRIs and CT scans) to identify patterns related to diseases. For instance, GANs have been used for image reconstruction, synthesis, segmentation, registration and classification [5, 9, 37, 39]. Moreover, GANs can be used to generate synthetic medical images that can be used to train machine learning models for image-based diagnosis or augment medical datasets. LLMs can enhance the output of multiple CAD networks, such as diagnosis networks, lesion segmentation networks and report generation networks, by summarising and reorganizing the information presented in natural language text format. This can create a more user-friendly and understandable system for patients compared to conventional CAD systems.
EHRs and other patient records are rich repositories of data, and LLMs can be used to analyse these records in a sophisticated manner [40]. They can process and understand the information and terminology used in these records, which allows them to extract and interpret complex medical information. This capability extends beyond simple keyword matching, as LLMs can infer meaning from incomplete information, and even draw on a vast medical corpus to make sense of the data. Moreover, LLMs can integrate and analyse information from multiple sources within the EHR. They can correlate data from lab results, physician’s notes and medical imaging reports to generate a more holistic view of the patient’s health [10]. This can be particularly useful in complex cases where the patient has multiple conditions or symptoms that may be related.
LLMs, like GPT-4, have shown medical knowledge despite lacking medicine-specific training [10, 29]. One of the most impressive aspects of these models is their ability to apply this knowledge in decision-making tasks [10]. For example, when presented with a hypothetical patient scenario, an LLM can generate a list of potential diagnoses based on the symptoms described, suggest appropriate tests to confirm the diagnosis and even propose a treatment plan. In some studies, these models have shown near-passing performance on medical exams, demonstrating a level of understanding comparable to that of a medical student [29]. However, limits exist, and the models’ outputs may carry certain risks and cannot fully substitute outpatient physicians’ clinical judgement and decision-making abilities [14].
LLMs such as GPT-4 and PALM-2 can be used to generate summaries of patient data [41]. This could be particularly useful in healthcare settings where large amounts of data are collected and need to be interpreted quickly and accurately. For instance, an EHR may contain patient data such as medical history, medications, allergies and laboratory results. A generative AI model could be trained to read through this data, understand the key points and generate a concise summary. This summary could highlight critical information such as diagnosis, prescribed medications and recommended treatments. It could also identify trends in the patient’s health over time. By automating this process, healthcare providers could save time and ensure that nothing important is overlooked. Furthermore, these summaries could be used to improve communication between different healthcare providers and between providers and patients, as they provide a clear and concise overview of the patient’s health status. The ability of LLMs to automate such processes can alleviate the current documentation burden and the consequent burnout many physicians across the world face [41]. Currently, many clinicians, due to organisational policies or health insurance requirements, are required to fill in lengthy documentation beyond what is required for routine clinical care. Studies have shown that many physicians spend over 1 h of time on electronic health record tasks for every hour of direct clinical face time [42]. Additionally, the cognitive load and frustration associated with documentation can reduce work satisfaction. contributing to their burnout [43]. Implementation of natural language processing tools to automate documentation could lessen this burden. An LLM embedded in the relevant information platform can undertake the documentation and provide draft versions for the clinician to approve [40, 41]. For example, hospitals can use LLMs to generate routine progress notes and discharge summaries [44].
Further to this, there is potential for these LLM-based applications to reduce medical errors and capturing missed information by providing a layer of scrutiny when embedded in EHRs [45]. In addition to automating documentation, LLMs integrated into EHRs could help reduce medical errors and ensure important information is not missed. Studies have found that many hospital patients will experience a preventable medical error, often due to issues like misdiagnosis, prescription mistakes or examination findings that are not followed up correctly [46]. Also, LLMs have the potential to serve as a decision support tool by analysing patient charts and flagging discrepancies or gaps in care [45]. For example, an LLM could cross-reference current symptoms and diagnostics against past medical history to prompt physicians about conditions that require further investigation. Additionally, they could scan medication lists and warn of potential adverse interactions or contraindications.
Generative AI can also be used to automate routine tasks in healthcare, such as scheduling appointments, processing claims and managing patient records [47]. For example, AI models can be used to develop intelligent scheduling systems. These systems can interact with patients through chatbots or voice assistants to schedule, reschedule or cancel appointments. They can consider factors such as doctor’s availability, patient’s preferred time and urgency of the appointment to optimize the scheduling process. Generative AI can also automate the process of insurance claims. It can read and understand the claim documents, verify the information, check for any discrepancies and process the claim. This can significantly reduce the time taken to process claims and minimise errors. By automating these tasks, healthcare providers can save time and resources and improve the patient experience as they get faster responses and more efficient service.
Generative AI can analyse a patient’s genetic makeup, lifestyle and medical history to predict how they might respond to different treatments [48]. This is achieved by training the AI on large datasets of patient information, allowing it to identify patterns and correlations that might not be immediately apparent to human doctors. For example, the AI might notice that patients with a certain genetic marker respond particularly well to a specific medication. This information can then be used to create a personalized treatment plan that is tailored to the individual patient’s needs. This approach can lead to more effective treatment, as it considers the unique factors that might affect a patient’s response to medication. It can also lead to improved patient outcomes, as treatments can be optimized based on the AI’s predictions [48].
Generative AI can also be utilised in the field of mental health, particularly in the creation of interactive tools for cognitive behavioural therapy (CBT) [49, 50]. CBT is a type of psychotherapy that helps patients manage their conditions by changing the way they think and behave. Generative AI can be used to create personalized scenarios and responses that are tailored to the individual patient’s needs. For example, the AI might generate a scenario that triggers a patient’s anxiety, and then guide the patient through a series of responses to help them manage their reaction. This can provide patients with a safe and controlled environment in which to practice their coping strategies, potentially leading to improved mental health outcomes.
In the context of medical education and training, this technology can be used to generate a wide variety of virtual patient cases. These cases can be based on a diverse range of medical conditions, patient demographics and clinical scenarios, providing a comprehensive learning platform for medical students and healthcare professionals [51, 52]. One of the primary benefits of using generative AI in medical education is the ability to create a safe and controlled learning environment. Medical students can interact with these virtual patients, make diagnoses and propose treatment plans without any risk to real patients. This allows students to make mistakes and learn from them in a low stake setting. Generative AI can also create patient cases that are rare or complex, giving students the opportunity to gain experience and knowledge in areas they might not encounter frequently in their clinical practice. This can be particularly beneficial in preparing students for unexpected situations and enhancing their problem-solving skills. Furthermore, the use of AI in medical education can provide a more personalized learning experience. The AI can adapt to the learning pace and style of each individual, presenting cases that are more relevant to their learning needs. For example, if a student is struggling with a particular medical condition, the AI can generate more cases related to that condition for additional practice.
In addition to creating virtual patient cases, generative AI can also be used to simulate conversations between healthcare professionals and patients [51, 52]. This can help students improve their communication skills and learn how to deliver difficult news in a sensitive and empathetic manner. Moreover, the integration of AI in medical education can provide valuable data for educators. The AI can track the performance of students, identify areas of improvement and provide feedback, helping educators to refine their teaching strategies and curricula.
Generative AI can be used for patient education in several ways [35, 41]. It can be used to create personalized educational content based on a patient’s specific condition, symptoms or questions. For example, if a patient has diabetes, the AI can generate information about managing blood sugar levels, diet, exercise and medication. Generative AI can also engage patients in interactive learning experiences. Patients can ask questions, and the AI can generate responses, creating a dialogue that helps the patient understand their condition better. This can be particularly useful for patients who may be shy or embarrassed to ask certain questions to their healthcare providers. Furthermore, generative AI can also create visual aids, such as diagrams or infographics, to help patients understand complex medical concepts. For example, it could generate a diagram showing how a particular drug works in the body.
Generative AI can be programmed to generate content at different reading levels, helping to improve health literacy amongst patients with varying levels of education and comprehension [53]. It can also be used to create follow-up educational content and reminders for patients. For example, it could generate a series of emails or text messages reminding a patient to take their medication, along with information about why it is important. In addition, generative AI can be used to provide mental health support, generating responses to patients’ concerns or anxieties about their health conditions. This can help patients feel more supported and less alone in their health journey. Finally, generative AI can generate educational content in multiple languages, making healthcare information more accessible to patients who do not speak English as their first language.
The translational path of generative AI in healthcare is a journey that involves the integration of this advanced technology into the clinical setting [54]. This process has the potential to revolutionize the way healthcare is delivered, by automating tasks and generating relevant information, thus enhancing the efficiency of healthcare delivery [26, 35]. Generative AI can automate routine tasks such as data entry, appointment scheduling and even some aspects of patient care like monitoring vital signs or administering medication. This automation can free up a significant amount of time for clinicians, allowing them to focus more on direct patient care. By reducing the administrative burden on healthcare providers, generative AI can help improve the quality of care and increase patient satisfaction [41, 53]. In addition to automating tasks, generative AI can also generate relevant information for clinicians. For example, it can analyse patient data to predict health outcomes, identify potential health risks and suggest personalized treatment plans. This ability to generate insights from data can help clinicians make more informed decisions about patient care, potentially leading to improved patient outcomes.
However, the accuracy and completeness of the information generated by AI are crucial. Inaccurate or incomplete information can lead to misdiagnosis or inappropriate treatment, which can harm patients [14, 55]. Therefore, it is essential to ensure that the AI systems are well designed and thoroughly tested to produce reliable results. Despite the potential benefits, adopting generative AI in clinical medicine is not a straightforward process. It requires careful planning and execution [56]. This includes understanding the needs of the healthcare providers and patients, selecting the right AI technology, integrating it into the existing healthcare systems and training the staff to use it effectively. Moreover, there are also legal and ethical considerations, such as data privacy and security, that need to be addressed. Furthermore, it is important to manage expectations about what generative AI can and cannot do. Clinicians’ expertise and their ability to empathize with patients are still crucial in providing high-quality care.
The successful translation of generative AI into clinical practice hinges on thoughtful adoption strategies grounded in implementation science. Two models offer robust scaffolds: the technology acceptance model (TAM) at the individual user level [57] and the Non-Adoption, Abandonment, Scale-up, Spread and Sustainability (NASSS) framework for organisational integration [58]. Grounded in sociopsychological theory, TAM provides an evidence-based model for how end-user perceptions shape acceptance of new technologies like generative AI [59]. Its core tenets posit that perceived usefulness and perceived ease of use prove most determinative of uptake. TAM offers a quantifiable approach for predicting and influencing adoption that deployment efforts must consider. Segmenting staff and assessing beliefs allows tailored interventions addressing barriers like skills gaps, engagement, workflow integration and demonstrable benefits. Equally crucial, the NASSS framework delivers a holistic methodology assessing multi-level variables implicated in successfully embedding innovations. Its seven critical domains encompass technology design, value propositions, adopter priorities, organisational dynamics, wider contextual factors and their complex interplay [58]. Together, these lenses reinforce introduced generative AI responsibly, monitor progress and recalibrate based on emerging feedback. Melding TAM and NASSS perspectives provides a powerful blueprint for thoughtfully ushering generative AI into the twenty-first-century healthcare. They bring implementable strategies for the sociotechnical transition such innovations necessitate, promoting buy-in, facilitating integration, delivering sustained value and ultimately transforming care.
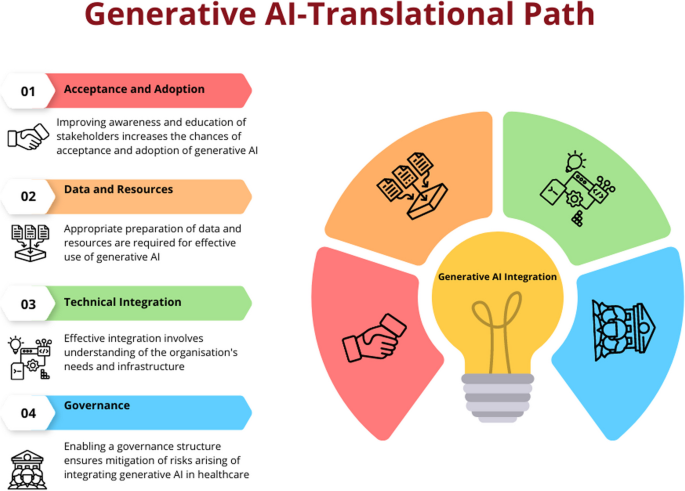
Based on these frameworks, the below content discusses the key components or steps a healthcare organisation or service should consider in integrating generative AI in their service delivery. The description will enable partners as to how to prepare their organisations and workforce to adopt and integrate generative AI to enable optimal care delivery. However, the description does not cover wider policy and legislative aspects that are required to facilitate the introduction of generative AI to healthcare. These characteristics are unique to various jurisdictions and continue to evolve rapidly, therefore are considered beyond the scope of this article.
The successful implementation of AI in healthcare hinges on the understanding and acceptance of its applications by end users [54], including medical professionals and patients. This comprehension fosters trust in AI systems, enables their effective use and aids in navigating ethical and regulatory challenges. Moreover, a solid grasp of AI promotes continuous learning and adaptation to the evolving landscape of AI technology. Therefore, investment in improving awareness for all partners is crucial to ensure the effective adoption and utilisation of AI in healthcare.
Utilising the TAM and NASSS frameworks to the implementation generative AI in healthcare involves consideration of the following components:
In addition to these factors, the model also suggests that external factors like social influence and facilitating conditions can influence the acceptance and use of a new technology [57, 59]. In the case of generative AI in healthcare, these could include regulatory approval, ethical considerations, patient acceptance and the overall healthcare policy and economic environment.
Adopting generative AI involves preparing data and resources within an organisation to effectively utilise this technology. This is a complex process requiring a systematic and strategic approach that involves several key steps.
Factors that impact computational requirements include model size, training data volume and speed of iteration desired. For example, a firm aiming to train a model with over a billion parameters on tens of billions of text examples would likely pursue a high-performance computing cluster or leverage cloud–based machine learning platforms. The precise hardware configuration—including GPUs/TPUs, CPUs, memory, storage and networking—scales with the model architecture and training plan [63].
Ongoing model development and fine-tuning also necessitates available compute. Organisations can choose between continuing to allocate internal resources or outsourcing cycles via cloud services [63]. Budgetary planning should account for these recurring compute demands if continually enhancing in-house LLMs is a priority. Overall, while leveraging external LLMs can minimise infrastructure investments, serious internal LLM initiatives can rival the computational scale of industrial research labs.
Integrating generative AI into a healthcare information system or platform can bring numerous benefits, such as improved disease diagnosis, enhanced patient monitoring and more efficient healthcare delivery. However, generative AI technologies like GANs and LLMs are complex to understand and implement [8]. The technology’s maturity, reliability and ease of integration into existing systems are crucial factors affecting its adoption [58]. Therefore, integrating generative AI into a hospital or healthcare information system involves several steps ranging from understanding the needs of the system to implementing and maintaining the AI solution. The first step in integrating generative AI into a healthcare system is to identify the focus area of implementation [62]. This could be anything from improving patient care, streamlining administrative tasks, enhancing diagnostic accuracy or predicting patient outcomes. Once the need is identified, the right AI model needs to be chosen. Generative AI models, such as GANs, can be used for tasks like synthesising medical images or generating patient data [6, 37). LLMs can be used for EHR analysis and as a clinical decision support tool [40]. Once the model is chosen, it needs to be trained on the collected data. This involves feeding the data into the model and adjusting the model’s parameters until it can accurately predict outcomes or generate useful outputs.
Once the AI model is trained and tested, it can be integrated into the healthcare information system [56, 62]. This involves developing an interface between the AI model and the existing system, ensuring that the model can access the data it needs and that its outputs can be used by the system. Developing such an interface or API allows the generative AI models to be seamlessly integrated into the organisational or clinical workflow. After integration, the AI system needs to be extensively tested to ensure its functionality, usability and reliability.
Regular maintenance is also necessary to update the model as new data becomes available and to retrain it if its performance drops [56, 62]. Furthermore, gathering regular/scheduled feedback from healthcare professionals will ensure the organisation can make necessary refinements to improve the system’s performance.
When leveraging external LLMs for healthcare applications, stringent data governance practices are imperative to safeguard sensitive patient information [64]. As text or speech data gets routed to third-party LLM services for analysis, the contents contain protected health information (PHI) and personally identifiable information (PII) that must remain confidential.
While LLMs themselves are static analysis models rather than continuously learning systems, the vendors hosting these models and powering predictions still physically or computationally access submitted data [65, 66]. Irrespective of the vendors’ reassurances about privacy commitments, obligations and restrictions on ingesting customer content for model retraining, residual risks of data leakage or unintended retention persist. To mitigate these risks, comprehensive legal contracts between the healthcare organisation and LLM vendor are foundational to ensuring PHI/PII protection in accordance with health regulations. Business associate agreements, data usage agreements and master service provider contracts allow formally codifying allowable LLM data processing, storage, transmission and disposal protocols. Such contracts also establish liability and enforcement mechanisms in case of a breach attributed to the vendor, including notification, indemnification and restitution clauses. Strict access controls, encryption schemes, activity audit protocols and authorization procedures should complement these contractual protections. While LLMs themselves may not endlessly accumulate healthcare data like perpetually learning systems, due diligence around the long-term fate of data sent to LLM prediction services remains highly advisable for risk-averse legal and compliance teams [14]. Establishing robust data governance for emerging clinical LLM integration can prevent problematic regulatory, ethical and reputational exposure [64].
While beyond the scope of this article to discuss in detail, the organisation will additionally have a responsibility to ensure the AI system complies with relevant healthcare regulations and privacy laws [55], such as Health Insurance Portability and Accountability Act (HIPAA) in the USA or General Data Protection Regulation (GDPR) in the European Union.
While generative AI has several potential applications in clinical medicine, there are also several challenges associated with its implementation. Some of the challenges include the following:
To minimise risks arising from the application of generative AI in healthcare, it is important to establish a governance and evaluation framework grounded in implementation science [64]. Frameworks such as the NASSS framework and the TAM should inform subsequent steps to promote responsible and ethical use of generative AI [58, 69]. This implementation science informed approach includes several steps to ensure appropriate testing, monitoring and iteration of the technology. The NASSS framework provides a useful lens for assessing the complex adaptive systems into which generative AI solutions would be embedded [58]. This framework examines factors like the condition, technology, value proposition, adopter system, organisation, wider context, and interaction and mutual adaptation over time. Analysing these elements can reveal barriers and enablers to adopting generative AI across healthcare organisations. Similarly, the TAM focuses specifically on human and social factors influencing technology uptake [59]. By evaluating perceived usefulness and perceived ease of use of generative AI systems, TAM provides insights into how both patients and providers may respond to and interact with the technology. TAM encourages stakeholder participation in system design to optimize user acceptance.
Both NASSS and TAM demand a thoughtful change management strategy for introducing new technologies like generative AI. This means conducting iterative testing and piloting of systems, co-developing governance policies with diverse voices, emphasizing transparency, providing extensive user training resources, developing protocols to assess AI quality and fairness, allowing user customization of tools, and continually evaluating impact to enable appropriate adaptation over time. Drawing from these models ensures responsible and ethical integration guided by end-user needs. The following are corresponding steps:
For each plausible risk, the assessment calibrates probability and severity estimates for variables like user types, information classes and mitigating controls. Continuous risk monitoring based on leading indicators and usage audits ensures the initial assessment adapts alongside inevitable model and application changes over time. Periodic probabilistic modelling using safety assurance methodologies further reinforces responsible governance. Overall, a nimble quantified risk approach prepares organisations to responsibly pursue generative AI’s benefits while protecting patients.
Healthcare systems worldwide face crises of affordability, access and inconsistent quality that now endanger public health [71]. Generative AI presents solutions to begin rectifying these systemic failures through responsible implementation guided by scientific best practices.
Validated frameworks like the TAM and NASSS model provide actionable roadmaps for change management, stakeholder alignment and impact optimization [58, 59]. They allow anticipating adoption barriers related to perceived value, usability, risks and more while delineating interventions to drive acceptance. With meticulous planning grounded in evidence, generative AI can transform productivity, insight and care enhancement. Use cases like workflow and documentation automation, personalized predictive analytics, and patient education chatbots confirm vast potential [26, 41, 45], provided the technology supports rather than supplants human expertise. Structured integrations emphasizing clinician control safeguard quality while unlocking efficiency. Thoughtful translation is essential, but implementation science provides proven guidance.
The time for debate has passed. Patients worldwide stand to benefit, and responsible leaders must act urgently. Strategic pilots, iterative scaling and governance emphasizing ethics alongside innovation will realize long-overdue progress. Generative AI cannot single-handedly fix broken systems, but carefully facilitated adoption can catalyse reform while upholding healthcare’s humanitarian obligations. The approach, not just technology, defines success. Guided by wisdom and compassion, generative AI may help restore healthcare ideals so many now lack: quality, affordability and humane care for all.
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.